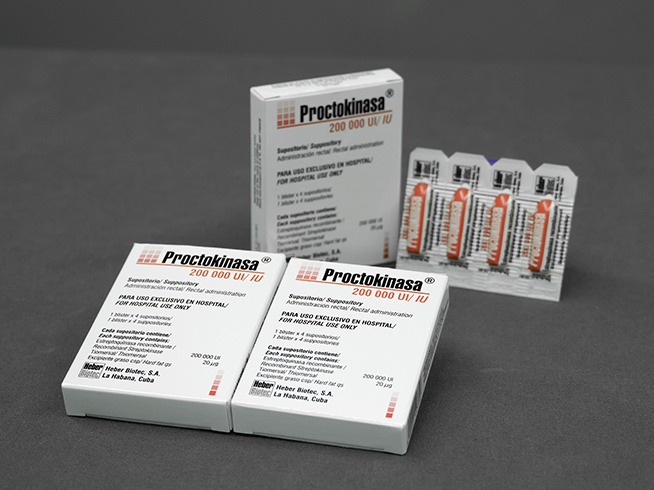
Proctokinasa®
Suppository containing recombinant streptokinase for the treatment of acute hemorrhoidal disease. Proctokinasa® is a non-invasive treatment for acute hemorrhoidal disease. Compared to other products frequently used in medical practice, Proctokinase® achieves a better therapeutic response. In a few days the inflammation, perianal pain and rectal bleeding disappear. Proctokinasa® removes thrombi and microthrombi from the inflammatory process. Proctokinase® avoids invasive procedures such as thrombectomy.
Suppository
200,000 IU / 2 g
- Heat-sealable preformed socket blister with 4 suppositories of 200,000 IU of recombinant streptokinase
- Box containing a PVC / LDPE blister for 4 suppositories
- Box containing one aluminum / LDPE blister for 4 suppositories
Each suppository (2 g) contains 200,000 IU recombinant streptokinase; thiomersal 20 µg.
Store and transport at 2 to 8 ° C. Do not freeze.
Proctokinasa® is indicated for people over 18 years of age, with a clinical diagnosis of acute hemorrhoidal disease (flux [crisis] or hemorrhoidal thrombosis), generally characterized by sudden onset perianal pain , with tumors of variable size and appearance, which can be red-purplish, associated with anal edema, rectal bleeding, itching, a sensation of mass, constipation, among other signs and symptoms.
Hypersensitivity to the product (streptokinase) or to any of the components of the formulation (thiomersal, salicylates, including aspirin).
Streptococcal infection (including rheumatism), active internal bleeding (less than 3 weeks) or other conditions where there is a high risk of bleeding. Infection difficult to treat due to its location (recent prescription of anticoagulants, menorrhagia and menstrual period, hemorrhagic diathesis, erosive ulcer lesions of the digestive tract, recent major surgery (less than 14 days). No studies have been performed on the application of the Proctokinase® in children under 18 years of age, during pregnancy, or during breastfeeding. Its use is not recommended in these populations.
It should only be used under medical prescription. Contains thiomersal: may cause allergic reactions.
The product is safe and tolerable. The main adverse effects reported after its use are not causally related to the product (Proctokinase®). They are related to the clinical manifestations present in hemorrhoidal seizures (fluxion) and thrombosis (pain, anal itching and burning, sensation of mass, constipation, among others), mostly of mild intensity, which resolve spontaneously without drug treatment.
Remove the suppository from the blister and, holding it on the opposite side to the pointed end, carefully insert it through the anus. Administer rectally a suppository every 8 hours until completing 4 units. If necessary, treatment can be continued with additional units, up to a maximum of 6 to 8 suppositories.
The use of antifibrinolytics inhibits the action of thrombolytics.
The use of Proctokinasa® is not recommended during pregnancy and lactation.
They have not been reported.
An overdose of Proctokinase® increases the risk of rectal bleeding.