Heberprot-P®
- Drug containing recombinant human epidermal growth factor for effective healing of diabetic foot ulcers
- The only therapy in the world for the effective healing of deep and complex neuropathic and ischemic diabetic foot ulcers.
- Heberprot-P® infiltrates into diabetic foot ulcers to stimulate and accelerate progressive and sustained healing, and reduce the risk of lower limb amputation.
- Heberprot-P® is an injectable formulation, designed for an unmet medical need.
Treatment results with Heberprot-P®
83.9%
Lesion covered by granulation tissue
46.2%
With complete closure of the lesion
71%
Non-amputee patients
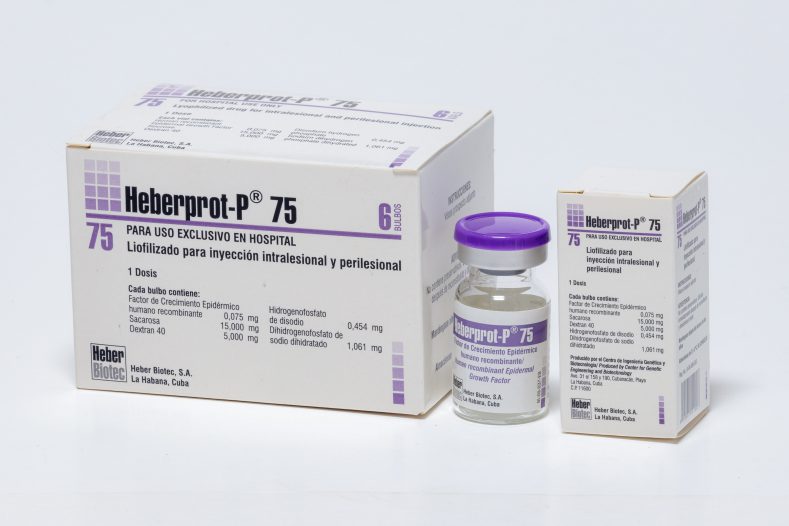
Lyophilisate for intralesional injection
75 µg / bulb
- Case with lyophilisate bulb + solvent vial + 1 sterile 5 mL syringe with 23G x 1½ ″ needle + 2 sterile 24G x 1½ ″ or 24G x 3/4 ″ needles + 2 sterile 26G x ½ needles ″
- Kit of 1 bulb with lyophilized recombinant human epidermal growth factor (HRF) 75 μg
- Kit of 6 bulbs with lyophilized recombinant human epidermal growth factor (HRF) of 75 μg
Each lyophilisate bulb contains 75 μg recombinant human epidermal growth factor (HRF); sucrose 15,000 mg; 5000 mg of dextran 40; disodium hydrogen phosphate 0.454 mg; Sodium dihydrogen phosphate dihydrate 1,061 mg. Each vial of solvent contains water for injections.
Temperatures between 2 and 8 ° C. Do not freeze.
Heberprot-P® should only be used under a medical prescription. It is indicated, together with other conventional therapies, for the healing of neuropathic and ischemic diabetic foot ulcers, with an area greater than 1 cm2, to stimulate the formation of useful granulation tissue, which allows closure by second intention or by autograft skin, as shown in several clinical studies.
Heberprot-P® is contraindicated in:
- Patients with a history of hypersensitivity to the product or any of its components.
- Patients with acute cardiovascular events such as: acute myocardial infarction, severe angina pectoris, acute stroke or transient ischemia or thromboembolic events in the previous three months.
- Patients with severe congestive heart failure (NYHA class III and IV), severe atrioventricular block (grade III), and uncontrolled rhythm atrial fibrillation.
- Patients with diabetic coma or diabetic ketoacidosis.
The administration of biological products must be careful and taking the necessary measures in case of unexpected adverse reactions. Before using Heberprot-P®, possible coexisting conditions such as infection and osteomyelitis should be treated. Adequate treatment of the infection of the lesion must be carried out before the use of Heberprot-P®.
In patients with severe ischemia due to peripheral macroangiopathy, a reperfusion procedure of the affected limb is recommended.
It is not known if Heberprot-P® passes into breast milk. It is not recommended for use in nursing mothers. Until now, there is not enough data to support its use in pregnant women and pediatric patients, so the doctor must make a risk-benefit balance in each case.
It should be administered with caution in patients with a personal history of acute cardiovascular events such as: acute myocardial infarction, cerebrovascular accident, or transient ischemia or thromboembolism; as well as in patients with clinically relevant valve disease (i.e. calcified aortic valves), severe arterial hypertension and a history of venous thrombosis.
It should be administered with caution in patients with renal failure with creatinine greater than 200 μmol / L, so the doctor must make a risk-benefit balance in each case.
In lesions suspected of malignancy, a biopsy should be performed to exclude the existence of a neoplasm, prior to the use of Heberprot-P®.
The treatment must be carried out by trained personnel with sufficient experience in the treatment of diabetic foot ulcers, who have the necessary diagnostic facilities. Heberprot-P® diluted solutions should be administered immediately after preparation. This medicine can only be used until the expiration date indicated on the bulb.
The most common side effects are mild and moderate: pain and burning at the injection site, chills, cold tremors, local infection and fever.
Heberprot-P® should always be used in conjunction with proper diabetic foot ulcer care, which consists of timely debridement of lesions, relief of pressure areas, and systematic care. The diagnosis and proper treatment of the ulcer infection must be carried out, prior to the use of Heberprot-P®.
Heberprot-P® will be administered at a rate of 75 µg diluted in 5 mL of water for injection, 3 times a week intralesionally. The administrations will be maintained until the complete granulation of the lesion is achieved, the closure of this by graft or a maximum of 8 weeks of treatment is reached.
Treatment should be discontinued in the event that useful granulation tissue is reached that covers the entire extent of the lesion, or in the event that the area is reduced to less than 1 cm2.
Infiltrations should be done after the lesions heal. The product is infiltrated into the edges of the ulcers with 26G x ½ ″ needles; and in the background, in the case of deep injuries, 24G x 1½ ″ or 24G x ¾ ″ needles should be used. The cleanest areas of the lesion should be infiltrated first, and the needle changed at the different puncture sites, in order to avoid the transmission of a possible infection from one site to another. Subsequently, the lesion should be covered with a gauze dressing moistened with saline solution, which keeps the environment moist and clean.
If after 3 weeks of continuous treatment, useful granulation tissue does not form in the ulcer bed, the treatment should be re-evaluated, and assess whether other factors that may hinder healing are influencing; among them, osteomyelitis, local infection or metabolic lack of control.
Interactions with other medications and other forms of interaction: It is not known if Heberprot-P® interacts with other topical medications, that is why it is recommended not to apply it with other topical products.
Pregnancy : There is not enough data to support its use in pregnant women.
Lactation : It is not known if Heberprot-P® passes into breast milk. Use in nursing mothers is not recommended.
Patients who are being treated with Heberprot-P® should not drive vehicles or machinery.
There have been no cases of overdose and no known antidotes for this product. Due to the local application of Heberprot-P® and the circulatory compromise of diabetic patients with advanced lesions, it is unlikely that systemic effects may occur.