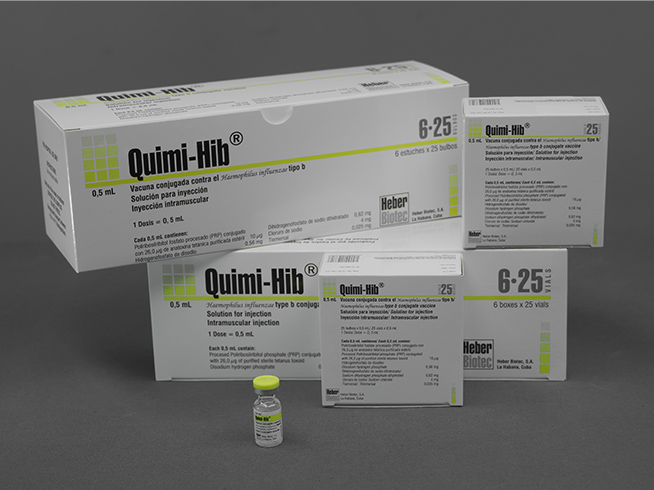
Quimi-Hib®
Conjugate vaccine for active immunization against diseases caused by Haemophilus influenzae type b (Hib), designed for children from 2 months to 5 years of age. Quimi-Hib® is safe, very well tolerated and little reactogenic. Its production meets the requirements of the World Health Organization.
Solution for intramuscular injection
10 µg
- Box with 25 vials containing a dose of 0.5 mL each
- Multiple box for 6 boxes with 25 vials containing a dose of 0.5 mL each
- Kit with a vial containing a dose of 0.5 mL
- Multi-kit for 10 kits with a vial containing a dose of 0.5 mL
- Multi-kit for 10 vials containing a dose of 0.5 mL each
Each dose (0.5 mL) contains:
- 10 µg of processed polyribosylribitol phosphate (PRP) conjugated to approximately 26 µg of sterile purified tetanus toxoid
- Sodium chloride 4 mg
- Anhydrous disodium hydrogen phosphate 0.56 mg
- Sodium dihydrogen phosphate dihydrate 0.62 mg
- Thiomersal 0.025 mg
- Water for injection q.s. 0.5 mL
Temperature from 2 to 8 °C. Do not freeze.
Quimi-Hib® is indicated for the active immunization of children from 2 months to 5 years of age, against diseases caused by Haemophilus influenzae type b (Hib), such as meningitis, epiglottitis, otitis, pneumonia, among others, which can cause disability and death.
Avoid vaccination in cases of known hypersensitivity to any of the components of the vaccine. Vaccination should be postponed in acute febrile illness.
As with any vaccine, a 1:1000 adrenaline solution or corticosteroids should be available for immediate use in cases of anaphylactic reactions. Individuals who develop hypersensitivity reactions after the administration of one dose of this vaccine should not receive the remaining doses. Administration of the vaccine in children affected by congenital or acquired immunodeficiency and in those undergoing immunosuppressive therapy may cause a limited or insufficient immune response.
Make sure the syringe needle does not penetrate any blood vessel. Do not administer intravenously. Once the product is administered, discard any remaining vaccine that is contained in the vial.
Quimi-Hib® is safe, very well tolerated and little reactogenic.
In controlled clinical studies in infants and older children vaccinated with Quimi-Hib®, it was determined that adverse reactions with a frequency greater than 1% in relation to the total doses administered are: low-grade fever (20.2%), pain at palpation at the injection site (2.2%) and fever greater than 38 ° C (1.3%). A group of adverse reactions were also found that occur with a much lower frequency (between 0.1% and 0.9% of the total doses administered), such as headache (0.8%), erythema at the injection site (0.8%) , irritability (0.5%), vomiting (0.5%), induration at the injection site (0.4%), rash (0.1%), drowsiness (0.1%), diarrhea (0.1%) and functional impotence (0.1%).
Adverse reactions basically appear in the first 72 hours after vaccination. They are mostly light in intensity and transitory in nature. In case of adverse reactions other than those indicated, you should consult your doctor.
The recommended vaccination schedule includes the administration of three doses of Quimi-Hib® from 2 months of age. The interval between doses is 2 months (8 weeks), and in no case less than 1 month.
In children who do not start the immunization schedule at 2 months of age, the following schedules are recommended, depending on age:
- For children between 2 and 6 months of age, the recommended vaccination schedule is the complete primary series of three immunizations separated by intervals between 1 and 2 months.
- In infants beginning the vaccination scheme between 7 and 11 months of age, an immunization scheme of only two doses will be used, with an interval of 1 to 2 months between them.
- Older children, who start the scheme Starting at 12 months of age, they will receive a single dose of the vaccine.
The application of a booster dose is recommended to all children who received the immunization schedule in their first year of life. This dose is applied between 15 and 18 months of age, as long as a minimum of 2 months has elapsed since the application of the last dose of the primary scheme.
In all cases, the scheduled dose can be administered up to ± 7 days from the corresponding date.
Quimi-Hib® should be administered intramuscularly in the anterolateral aspect of the thigh, in children under 2 years of age, and at the deltoid level in children over 2 years of age. To obtain the dose of the vaccine (0.5 mL), you must extract 0.5 mL of the solution, and inject.
Quimi-Hib® can be administered in different anatomical sites, with the vaccines against diphtheria and tetanus; diphtheria, tetanus and pertussis (DPT); and against hepatitis B. Simultaneous administration to the same anatomical site may be carried out by separating each inoculation at a distance greater than 1 inch and using different syringes.
Cases of overdose have not been studied.