Vaccine based on the receptor-binding domain (RBD) of the spike protein of the SARS-CoV-2 virus, its most protruding segment, and is intended to introduce antibodies that interfere with the entry of the pathogen into cells ; mechanism that is fundamental in all existing formulations of its kind to generate protection. This formulation is adjuvanted in aluminum hydroxide.
The selection of the yeast Pichia pastoris as an expression system was due to the experience of the CIGB in the use of the technological platform, which is cheaper in relation to others and, therefore, with the possibility of an advantage in terms of production costs.
Since RBD is a covalently associated glycoprotein, the saccharides of Pichia pastoris provide it with an immunopotentiating effect that favors immunogenicity. In fact, Abdala does not need a carrier protein to achieve high levels of this indicator, evaluated in phases I-II of a clinical study.
It was designed by means of protein engineering using structural bioinformatics computational methods aimed at increasing its similarity to the SARS-CoV-2 virus.
The protein created stands out for its versatility, as it can be used as an immunogen alone and in the form of a hybrid nanoparticle.
Phase III clinical study of Abdala
92.28%
Efficacy against symptomatic disease
100%
Efficacy in preventing severe systemic disease and death of vaccinated people
90%
Effectiveness in critically ill patients
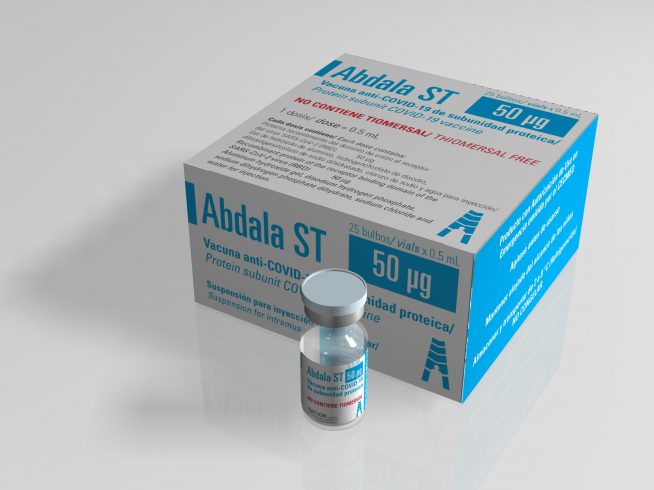
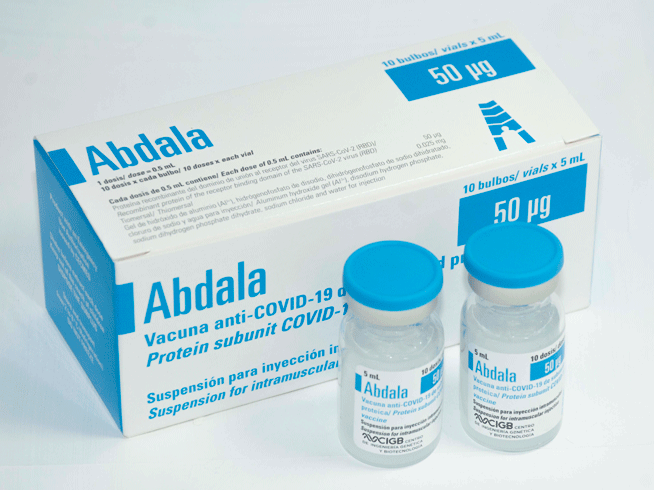
Suspension for injection
Intramuscular injection
- Box of 6 individual boxes containing 1 vial with 10 doses of 0.5 mL each
- Boxes of 10 vials containing 10 doses of 0.5 mL each
- Boxes of 25 vials containing 1 dose of 0.5 mL each
Each 0.5 mL contains:
- Recombinant protein of the SARS-CoV-2 virus receptor-binding domain (RBD) 0.05 mg
- Thiomersal 0.025 mg
- Aluminum hydroxide gel (Al³ +)
- Disodium hydrogen phosphate
- Sodium dihydrogen phosphate dihydrate
- Sodium chloride
- Water for injection, s.q.
The vaccine should be stored between 2 and 8 ºC. DO NOT FREEZE; DISCARD THE VACCINE IF IT FROZES.
- Hygienic-sanitary and biosafety measures will be taken to extremes during the inoculation of the product and for the elimination of the materials used.
A sterile syringe and needle should be used for each injection. - Upon completion of intramuscular application, discard bulb, needle, and syringe.
- Once the product has been administered, DO NOT recap the used needle. It must be disposed of in designated deposits.
- Multi-dose bulbs that have not been fully used in one immunization session CANNOT be kept for use in subsequent sessions and must be discarded at the end of the immunization session.
Posology
Three doses should be administered separated by 14-day intervals (0-14-28 days schedule) between each one.
Method of administration
- The product will be administered intramuscularly: 0.5 mL in the deltoid region of the arm (upper external part); preferably in the non-dominant arm.
- Immunizations will be carried out by trained nursing personnel and certified vaccinators for this activity.
- For the administration of the product, syringes of 1 mL volume, labeled with 10 divisions, and with 23 G or 22 G x 25 mm needles will be used.
- SHAKE GENTLY BEFORE REMOVING EACH DOSE.