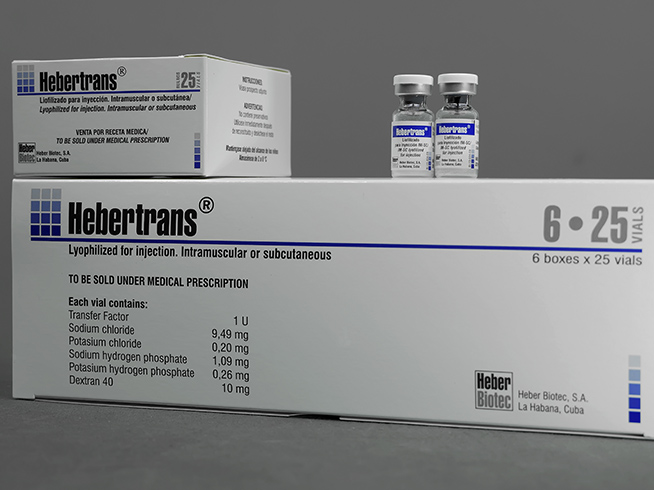
Hebertrans®
Drug containing transfer factor. Hebertrans® is useful for the treatment of patients with cellular immunodeficiency, herpes zoster, herpes simplex, cancer and diseases of allergic or autoimmune origin
Lyophilisate for intramuscular and subcutaneous injection
Transfer factor 1 U.
Case with 6 cases for 25 vials.
Each vial contains:
- Transfer factor 1 U (equivalent to 1 mg of protein)
- Sodium chloride 9.49 mg
- Potassium chloride 0.20 mg
- Disodium hydrogen phosphate 1.09 mg
- Potassium dihydrogen phosphate 0.26 mg
- 10 mg Dextran 40
Store at 2 to 8 ° C.
Hebertrans® injectable is useful for patients with:
In children with recurrent mucocutaneous herpetic infection and cellular immunodeficiency, the use of Hebertrans® has been beneficial as it has reduced the healing time of the lesions, of the frequency of recurrences and the normalization or improvement of the cellular immunity indicator parameters. Good evolution has also been reported in severe herpetic meningoencephalitis.
In herpetic keratitis, the use of Hebertrans® every other day, combined with antiviral therapy, in a double-blind study, produced a decrease in the frequency of appearance complications ( p = 0.08) (uveitis, corneal dystrophy) and reduction in the healing time of the lesions ( p = 0.002).
In children with atopic dermatitis, treatment with Hebertrans® caused significant clinical and laboratory improvement (11/12) in patients in whom the disease concurred with cellular immunodeficiency; but the effect was not evidenced in 10 patients with normal cellular immunity.
Good results have been reported after the use of Hebertrans® in patients with allergic keratoconjunctivitis.
Hebertrans® is contraindicated in patients with hypersensitivity to dextran or any of the salts present in the preparation.
There are no studies on the use of Hebertrans® in pregnant women. Therefore, the doctor must do a risk-benefit analysis in each case before using it.
Hebertrans® contains no preservatives. Use immediately after reconstitution. Should not be used if, once reconstituted, it presents precipitate, turbidity or color.
- Except for slight erythema at the injection site in 2% of cases, no significant side effect has been reported with the use of Hebertrans®.
- In children who have received Hebertrans® for more than 2 months, no adverse effects on growth or psychosomatic development have been found.
In adults, the most commonly used dose is 1 U. In children, the dose is 1 or 2 U/m2 of body surface.
The frequency of administration may vary depending on the diagnosis and evolution of the disease: between 1 U daily up to 7 days, and 1 to 2 U / week.
In some patients with chronic diseases, treatment can last up to 6 months.
There have not been reported to date.
- Use in pregnancy : There are no studies on the use of Hebertrans® in pregnant women. Therefore, its safe use during pregnancy has not been established and the doctor must do a risk-benefit analysis in each case before use.
- Pediatric use : Hebertrans® has been used in children with primary immunodeficiencies and recurrent infections, especially of the upper respiratory tract , herpes virus infections and others. No major side effects or disorders of growth or psychosomatic development have been reported, even after several months of continuous treatment.
They have not been reported to date.
Overdose of this product is not described. Toxicity studies in animals indicate that it is very low toxic even at doses of 3 U / kg, much higher than therapeutic doses in humans.