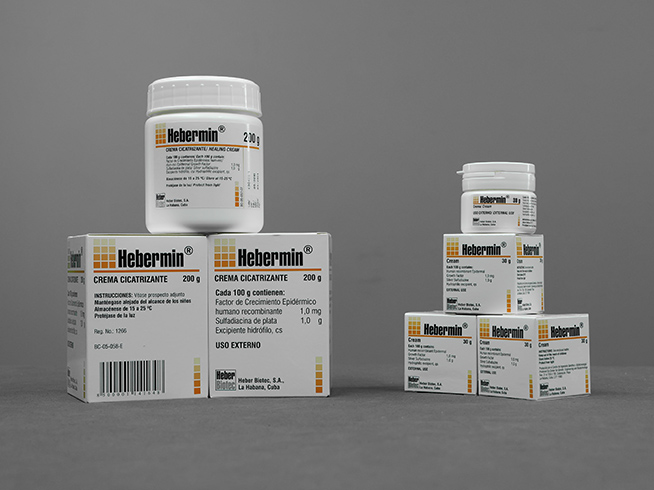
Hebermin®
Dermatological cream containing recombinant human epidermal growth factor, Hebermin® reduces the time of healing and epithelialization in burns, eschar and surgical procedures. Hebermin® regulates cell growth. It is an antimicrobial with bacteriostatic action.
Cream
10 μg of FCEhr/g of cream
- White-opaque polyethylene jars with a capacity of 30 & nbsp; g
- White-opaque polyethylene jars with a capacity of 200 g.
Each 100 g contains 0.001 g recombinant human epidermal growth factor (HRF); silver sulfadiazine 1.00 g; 4-methyl hydroxybenzoate 0.180 g; 4-propyl hydroxybenzoate 0.020 g.
Store below 25 °C. Protect yourself from light.
Hebermin® is used in the treatment of superficial and deep dermal and hypodermic burns, and bedsores. It can be used in other surgical procedures that require tissue healing or regeneration, such as radiation injuries, cytostatic extravasation ulcers and circulatory insufficiency ulcers, as well as in the prophylaxis of superficial radiotherapy injuries.
Hebermin® is contraindicated in patients with hypersensitivity or allergy to sulfonamides. It should not be used in newborns, or during pregnancy and lactation.
Hebermin® should be used with caution in patients with impaired liver and kidney function and in patients with glucose-6-phosphate dehydrogenase deficiency.
The surface where the cream is applied should not be exposed directly to the sun, as this alters the medicine. In such cases, the treated surface must be covered or bandaged.
Reactions characterized by intense burning that tends to disappear, rash, itching, and eventually crystalluria have been reported in patients with a large burned body surface in which silver sulfadiazine can be absorbed. Reactions similar to those produced by sulfo derivatives may occur in hypersensitive patients.
After cleaning the lesions, a thin layer is applied directly to the lesions. The dose is determined by the extent of the lesions. The healing of the lesions and the application of Hebermin® every other day is recommended. In infected lesions, local cleaning and complementary treatment with antibiotics are required.
Symptoms due to incompatibilities or interactions with other drugs have not been described.
It should not be used in women with full-term pregnancies, or newborns during the first month of life, since silver sulfadiazine can cause kemicterus.
They have not been described.
No overdose symptoms have been described.