Heberpenta®-L
Liquid pentavalent vaccine that contains antigens against diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae type b (Hib), widely used in other vaccines. Reduce the number of injections. It allows increasing coverage in vaccination programs. Reduces the risks of unsafe injections.
Suspension for intramuscular injection
Each 0.5 mL dose contains:
- Sterile purified diphtheria toxoid 25 Lf
- 10 Lf purified tetanus toxoid
- Whole and inactivated cells of Bordetella pertussis 16 UO
- Purified surface antigenic protein from hepatitis B virus 10 µg
- Haemophilus influenzae type b synthetic polyribosylribitol phosphate conjugate (PRP-TT) 10 µg
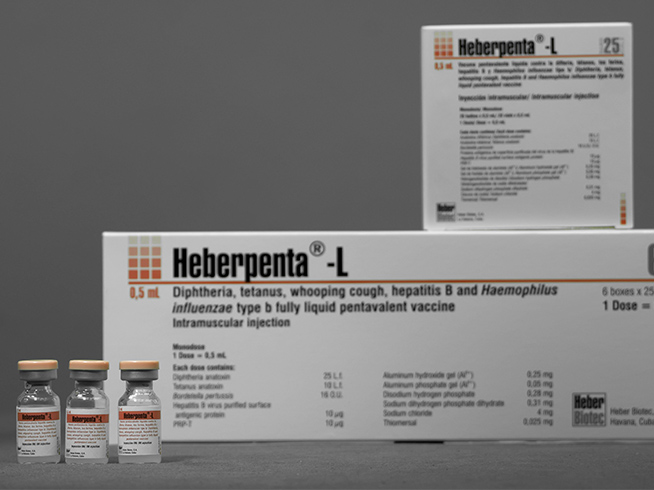
- Box with 25 bulbs containing a dose of 0.5 mL each
- Multiple box with 6 boxes of 25 bulbs containing a dose of 0.5 mL each
- Individual kit for a bulb containing a dose of 0.5 mL
- Multiple kit for 10 individual kits containing a dose of 0.5 mL each
- Case with 10 bulbs containing a dose of 0.5 mL each
- Box with 100 pre-filled syringes containing a dose of 0.5 mL each
Each 0.5 mL dose contains:
- Sterile purified diphtheria toxoid 25 Lf
- 10 Lf purified tetanus toxoid; whole and inactivated cells of Bordetella pertussis 16 UO
- Hepatitis B virus purified surface antigenic protein 10 µg
- Haemophilus influenzae type b synthetic polyribosylribitol phosphate conjugate (PRP-TT) 10 µg
- 0.25 mg aluminum hydroxide gel as adjuvant; 0.05 mg aluminum phosphate gel as adjuvant
- Disodium hydrogen phosphate 0.28 mg to maintain the ionic strength and pH of the medium
- 0.31 mg sodium dihydrogen phosphate dihydrate to maintain the ionic strength and pH of the medium
- Sodium chloride 4 mg to maintain the ionic strength and pH of the medium
- Thiomersal 0.025 mg preservative against microbial growth
- Water for injections q.s.p 0.5 mL
- Solvent
Temperature from 2 to 8 °C. Do not freeze.
Heberpenta®-L is indicated for active immunization against diphtheria, tetanus, pertussis ( Bordetella pertussis ), hepatitis B and diseases caused by Haemophilus influenzae type b (Hib), in children from 2 months of age.
Heberpenta®-L vaccine should not be administered to children over 7 years of age, or to adults, because reactions to diphtheria toxoid or the pertussis component may occur.
Heberpenta®-L should not be administered to people with known hypersensitivity to any of the components of the vaccine, or to people who have shown signs of hypersensitivity after previous administration of diphtheria, tetanus, antipertussis, anti-hepatitis B or anti- Haemophilus influenzae type b.
As with other vaccines, administration of Heberpenta®-L should be postponed in children with severe and acute febrile illnesses.
Heberpenta®-L is contraindicated if the child suffered from encephalopathy of unknown etiology, which appeared within 7 days of a previous vaccination with a vaccine containing pertussis. Under these circumstances, the vaccination cycle could be continued with DT, HB and Hib vaccines.
The possibility of allergic reactions should be evaluated in individuals sensitive to the components of the vaccine.
As with all vaccines, it is important to have an epinephrine hydrochloride solution (1: 1000) available for immediate use in the event of anaphylaxis or an acute post-vaccination hypersensitivity reaction. For this reason, it is advisable to keep the child under medical observation for 30 minutes after the administration of the vaccine.
The decision to administer subsequent doses of Heberpenta®-L vaccine should be carefully assessed and the risk-benefit ratio analyzed, in children with a history of some of the following reactions, within 48 hours after a dose of Heberpenta®-L:
- Temperature > 40 °C, not due to any other identifiable cause.
- Collapse or shock-like state (hypotonic-hyporesponsive episode).
- Persistent crying (lasting more than 3 hours).
This same assessment should be made if the child presented seizures with or without fever, within three days after the administration of a dose of Heberpenta®-L.
The contraindications adopted by the public health authorities of each country must reflect a balance between the risk of administering the vaccine and the risk of the disease, since the risk of the vaccine is extremely low compared to the risk of these diseases in many developing countries.
Overdose : Not described.
If Heberpenta®-L vaccine is used in children who at the time of immunization are or have recently received immunosuppressive therapy, or who have malignant diseases or their immune system is weakened from any cause , the expected immune response may not be obtained.
As with any vaccine, vaccination with Heberpenta®-L may not protect 100% of susceptible individuals.
The clinical experience with the Heberpenta®-L vaccine applied to infants shows that the highest proportion of adverse events occurs between 24 to 48 hours after the administration of the vaccine, mainly in the first 24 hours.
The frequency of recording adverse events is higher after the application of the first dose of the vaccination schedule and decreases after the application of subsequent doses.
There is a marked predominance of systemic adverse events over local effects at the injection site.
After the administration of Heberpenta®-L, the predominant adverse events are fever (axillary temperature greater than 37.5 ° C) and low-grade fever (axillary temperature between 37.1 and 37.4 °C), with a frequency of about 40% of all vaccinated.
Other systemic events that can also be registered after the administration of the vaccine are: irritability, loss of appetite, vomiting, diarrhea, drowsiness, rash, allergic reactions, paleness, sweating and coldness.
At the local level at the injection site, pain on palpation, erythema and induration are recorded with a frequency of around 1%, in relation to the total doses administered. These reactions are usually localized and do not require treatment. Severe local reactions and abscesses at the level of the inoculation site are very rare events, associated with poor technique in the application of the vaccine.
In general, mild adverse events predominate, which resolve in a short time without the need for treatment.
In post-vaccination follow-up visits to children with more severe adverse reactions, full recovery has been observed, without persistent sequelae.
For vaccines with the same components as Heberpenta®-L, other rare events have been described, such as hypotonia-hyporesponsiveness (collapse or a state similar to shock ), characterized by paleness, cyanosis, hypotonia and hyporesponsiveness, which appears more frequently in the first 48 hours after the vaccine is administered. In clinical experience with Heberpenta®-L events of this type can occur with a very low frequency (less than 0.005%):
- Persistent crying (lasting > 3 hours), is recorded with a frequency less than 0.05%.
- Fever equal to or greater than 40 °C is recorded with a frequency less than 0.005%.
- Seizures can occur, generally associated with the presence of fever (less than 0.005%).
- It has been reported that anaphylactic reactions appear very rarely (around of 1 case per million doses applied).
Heberpenta®-L is available in bulbs or pre-filled syringes containing a 0.5 mL infant dose. Each dose consists of 25 Lf of diphtheria toxoid, 10 Lf of tetanus toxoid, 16 IU of whole and inactivated Bordetella pertussis cells, 10 μg of PRP-T and 10 μg of virus surface antigen of hepatitis B.
Dose
A 0.5 mL dose of Heberpenta ® -L vaccine should be administered.
Route of administration
Heberpenta ® -L is administered by deep intramuscular injection. The vaccine is administered to the middle third of the anterolateral outer thigh in children from 2 months of age, and to the deltoid muscle in children older than 24 months. It should not be given by intravenous injection.
Administration schemes
Primary vaccination with Heberpenta ® -L consists of three doses of the vaccine that are administered in the first 6 months of life.
The vaccine is given from 2 months of age. The recommended interval between doses is 8 weeks.
The recommended schedule is three 0.5 mL doses administered at 2, 4 and 6 months of age.
Additionally, in countries where it is considered convenient to administer the hepatitis B vaccine at birth, the recommended schedule will be one dose of the hepatitis B vaccine at birth, followed by the three doses of Heberpenta®-L administered at 2, 4 and 6 months of age.
Booster dose
It is recommended to apply a booster dose with the DPT and Quimi-Hib® vaccines, or with the Heberpenta®-L vaccine between 15 and 18 months of age.
Although there is still insufficient information on the Heberpenta®-L interaction with other vaccines, it is common in pediatric vaccination to simultaneously apply different vaccines that use the same administration schedule. In that case, vaccines should be administered separately, at different sites and with different syringes.
They have not been described.